A new rechargeable lithium-air battery potentially has four times greater energy density than a traditional lithium-ion battery.
June 4, 2025The Science
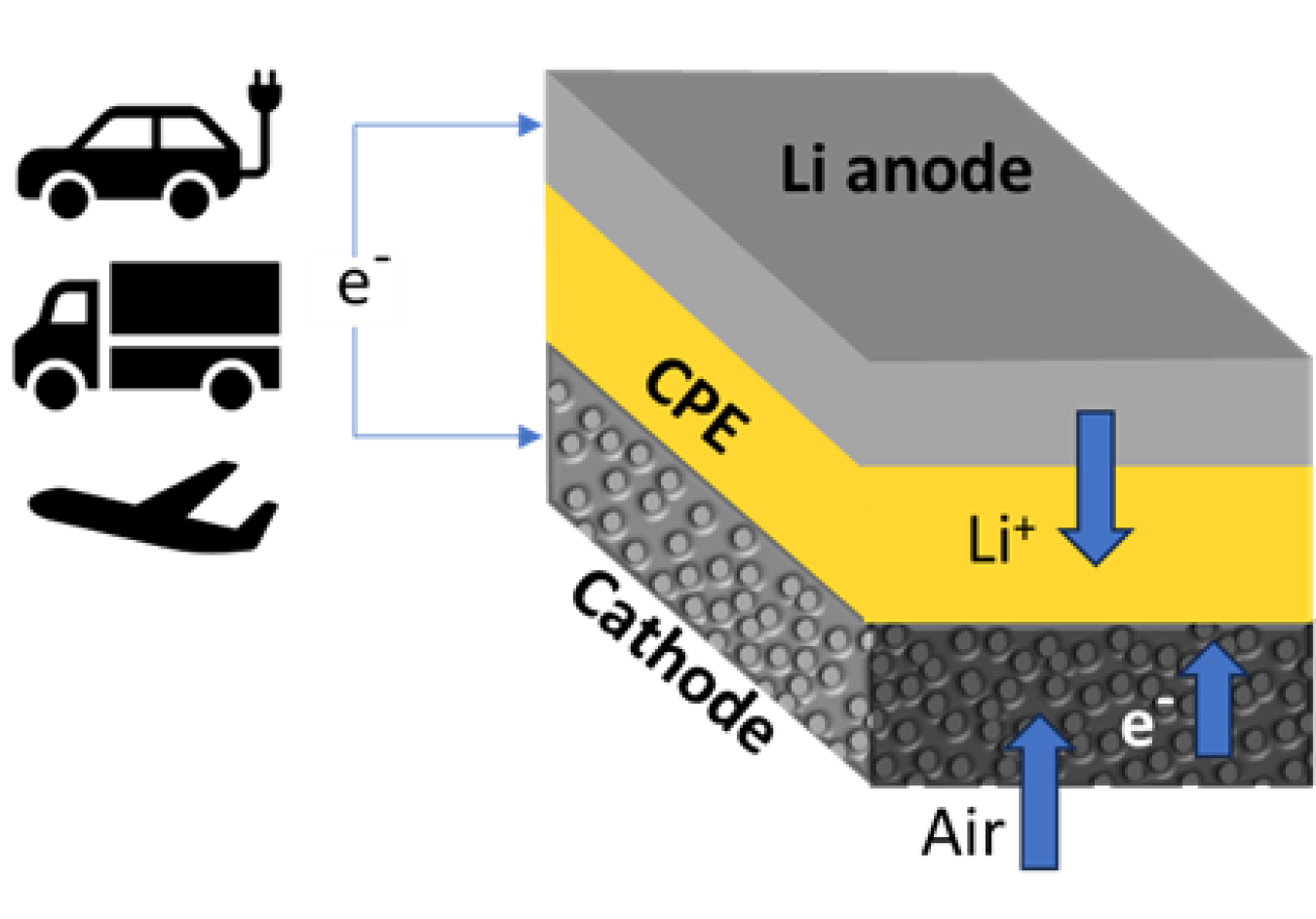
Researchers have designed a new lithium-air battery that can store much more energy per volume of battery than today’s lithium-ion designs. The new battery uses a solid composite electrolyte based on nanoparticles that contain lithium. The electrolyte is embedded in a matrix made of a special material called a ceramic-polyethylene oxide polymer. This approach differs from conventional batteries, where the electrolyte is a liquid. This solid-state lithium-air battery is the first to achieve a four-electron chemical reaction at room temperature. This is important because most lithium reactions involve one or two electrons. Reactions involving more electrons means more energy storage. Researchers showed that the battery can be recharged for at least 1,000 charge-discharge cycles. With further development, this lithium-air design could reach a record energy density of 1,200 watt-hours per kilogram. That density is four times greater than lithium-ion batteries.
The Impact
The lithium-air battery has the highest projected energy storage density of any technology being considered for the next generation of batteries. This technology would dramatically increase how much energy batteries can store. Using a solid-state electrolyte instead of a liquid electrolyte would also dramatically reduce safety concerns due to fire. Furthermore, this discovery opens up novel ideas for designing lithium-based battery chemistry that works at room temperature. These future designs could achieve even greater energy storage.
Summary
Developing a battery with an energy density comparable to that of gasoline is a long-sought goal in battery research and development. A lithium-air battery based on lithium oxide (Li2O) formation has such a theoretical potential. Lithium-air batteries offer great promise due to their high energy density and low cost. So far, lithium-air battery demonstrations have been limited to only one- or two-electron reaction processes, resulting in the formation of lithium superoxide (LiO2) or lithium peroxide (Li2O2), respectively.
Scientists at the Illinois Institute of Technology and Argonne National Laboratory have developed a new approach based on a four-electron reaction process to produce lithium oxide (Li2O) formation and decomposition, enabling the battery to deliver a much higher energy density compared to current Li-ion technology. The successful implementation of the four-electron reaction in the lithium-air battery relies on the utilization of a solid-state electrolyte combined with a catalyst, trimolybdenum phosphide (Mo3P). The composite electrolyte embedded with Li10GeP2S12 nanoparticles exhibits high ionic conductivity and stability and high cycle stability. Low-dose cryogenic transmission electron microscopy carried out at the Center for Nanoscale Materials, a Department of Energy Office of Science user facility, confirms the reaction mechanism, which favors four-electron reaction chemistry by reversible formation and decomposition of Li2O as the main product. The research found that the battery is rechargeable for at least 1,000 cycles at room temperature, which represents significant progress toward practical applications of lithium-air batteries.
Contact
Jianguo Wen
Argonne National Laboratory
jwen@anl.gov
Funding
This research was funded by the Department of Energy (DOE), including the DOE Office of Science, Basic Energy Science program’s Joint Center for Energy Storage Research (JCESR) Energy Innovation Hub; and by the Illinois Institute of Technology; the National Science Foundation; Northwestern University; the International Institute for Nanotechnology; the Keck Foundation; and the State of Illinois. The research used the resources of the Center for Nanoscale Materials, a DOE Office of Science user facility.
Publications
Kondori, A., et al., A room temperature rechargeable Li2O-based lithium-air battery enabled by a solid electrolyte. Science 379, 499-505 (2023). [DOI: 10.1126/science.abq1347]
Related Links
New design for lithium-air battery could offer much longer driving range compared with the lithium-ion battery, Argonne National Laboratory press release
Illinois Tech assistant professor publishes paper in Science on novel chemistry behind ultra-high power density batteries, Illinois Institute of Technology news