Researchers gain a new understanding of the binding chemistry of radioactive antimony, opening doors for targeted therapy.
Isotope R&D and Production (DOE IP)
January 5, 2026The Science
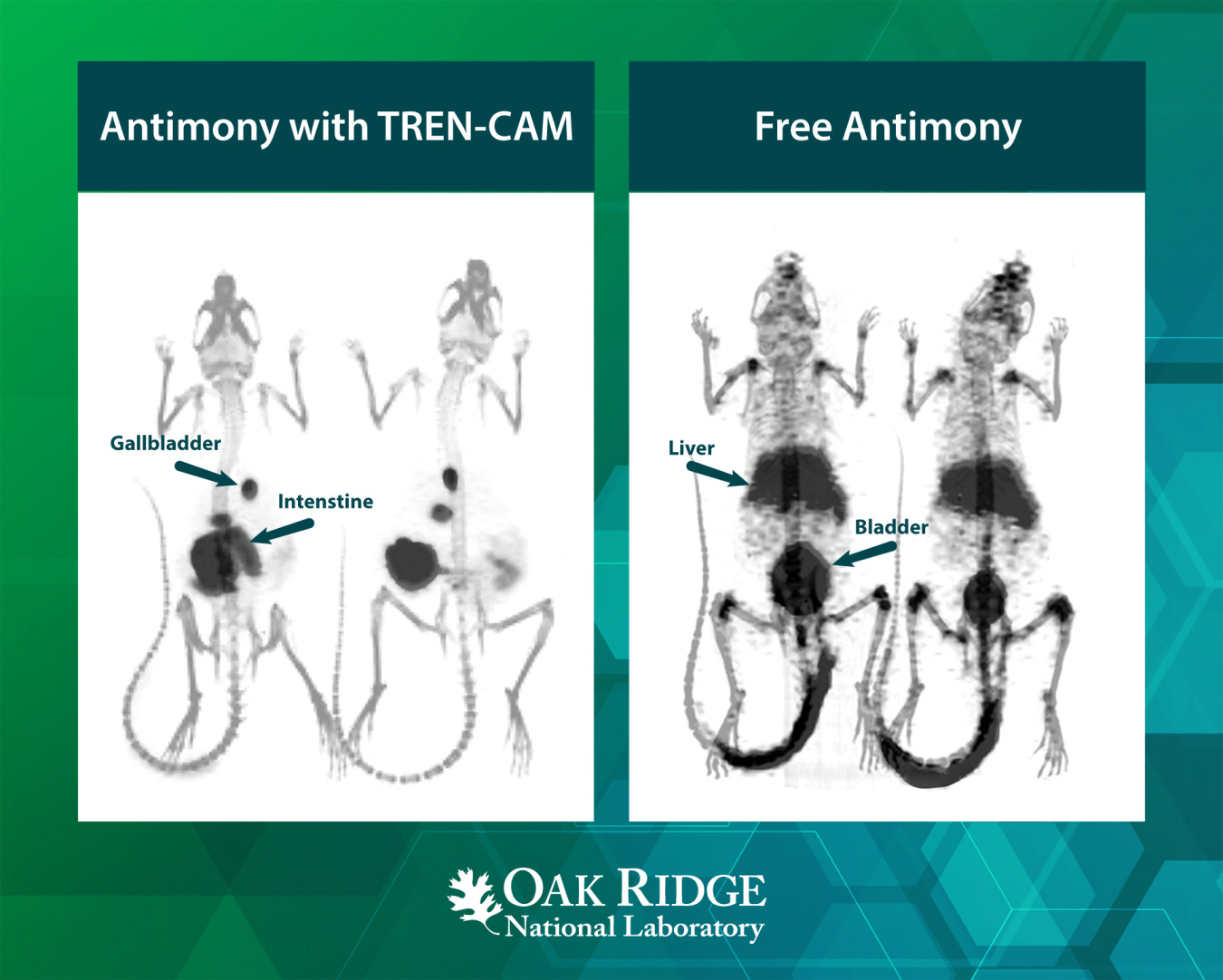
Researchers first recognized antimony-119 (Sb-119) more than two decades ago as a potentially promising radioactive isotope for targeted cancer therapy. However, the lack of binding molecules has limited its medical use. These molecules, called chelators, can tightly hold antimony in the body. Researchers recently identified a chelator called TREN-CAM that fits the bill. In this study, TREN-CAM forms a highly stable, protective molecular cage, or complex, around the radioactive Sb in human blood and in mice. These results will help advance Sb-119 for future medical applications.
The Impact
This discovery brings radioactive antimony one step closer to clinical use in cancer treatment. When Sb-119 undergoes radioactive decay, it emits low-energy electrons called Auger electrons. These electrons travel less than the length of a single cell in the body. As a result, treatments can use them to very precisely target and destroy cancer cells. Targeted therapy with isotopes like Sb-119 could lead to highly effective cancer therapies while minimizing toxicity and side effects to patients. The Sb–TREN-CAM complex is the most stable form of radioactive antimony ever reported under biological conditions.
Summary
Sb-119 holds great promise for treating cancer because it releases short-range electrons that can damage cancer cells. However, using it in medicine requires a stable carrier. In this study, scientists investigated a new approach to binding antimony to influence its chemical behavior. TREN-CAM is a chelator designed to securely hold metal ions and prevent them from breaking down or moving unpredictably in the body. When TREN-CAM binds with antimony, it creates a robust molecular cage that maintains control of the radioactive element inside living organisms. Through laboratory experiments and studies in mice, researchers confirmed that the antimony/TREN-CAM complex stays largely intact in living systems. They were able to see that organ uptake for the complex is different than organ uptake observed for antimony without the TREN-CAM molecule. This stability ensures that the radioactive material stays at the tumor site long enough to be effective. The ability to image antimony in the body makes it useful for tracking treatment progress. This breakthrough in chelation chemistry opens new possibilities for using radioantimony in nuclear medicine.
Contacts
Nikki A. Thiele
Oak Ridge National Laboratory
thielena@ornl.gov
Jonathan W. Engle
Associate Professor
University of Wisconsin Madison
jwengle@wisc.edu
Funding
This research was supported by the Department of Energy’s Office of Isotope R&D and Production, managed by the DOE Office of Science.
Publications
Olson, A. P., et al., Towards the stable chelation of radioantimony(V) for targeted Auger theranostics, Angew. Chem. Int. Ed. 64, 15 (2025). [DOI: 10.1002/anie.202423878]