Researchers gain new insights into a strong bond between the isotope astatine-211 and common chemicals, creating new possibilities for cancer treatment.
Isotope R&D and Production (DOE IP)
June 30, 2025The Science
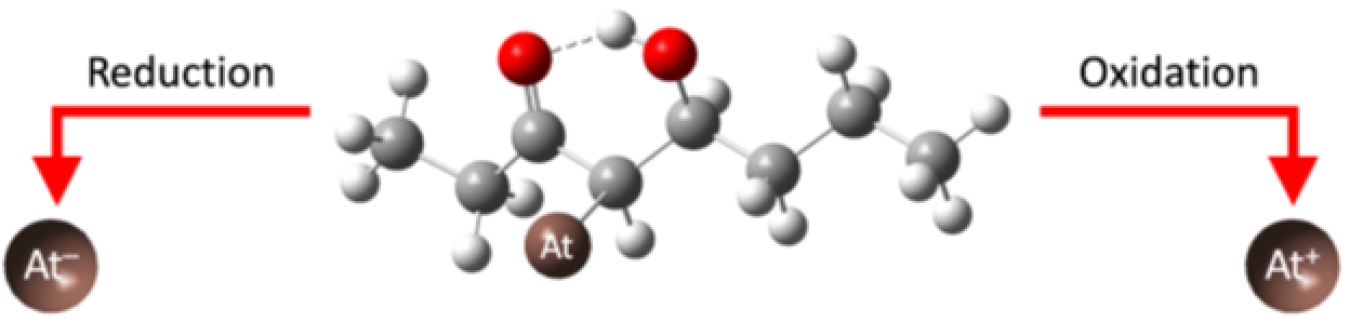
The half-life of astatine-211 (At-211) is less than 8 hours. This makes it one of the least abundant isotopes on Earth. At-211 is interesting because it emits alpha particles (the nuclei of helium atoms, which consist of two protons and two neutrons). As such, it is a promising agent for cancer therapy. Unfortunately, researchers know very little about its chemistry, including its reactivity and how it interacts with other atoms. In this research, scientists discovered how At-211 interacts with a class of chemicals known as ketones through the formation of covalent bonds. Covalent bonds are the strongest of all chemical bonds and are formed by two atoms that share their electrons equally. The discovery of the At-211-ketone interaction could improve cancer therapy drugs by providing a pathway to attach At-211 to cancer-targeting molecules.
The Impact
Targeted alpha therapy (TAT) is an important area of research for developing drugs to treat cancer. The idea behind TAT is to attach alpha-emitting isotopes, which can inflict high levels of cellular damage to tumors, to molecules that target cancer cells. The half-life of At-211 and its alpha particle decay makes it ideal for TAT drugs. It can inflict a large amount of damage without long-term side effects. The new discovery of a strong At-211-ketone bond opens the door for a new class of TAT drugs.
Summary
Building on previous work, researchers discovered a new interaction (covalent bond) of At-211 with a class of chemicals known as ketones. The researchers tested the strength of this bond using strong chemicals. The results indicate that the bond is very strong but can be broken with strong reducing or oxidizing agents. The formation of covalent bonds will allow At-211 to be tightly bound to cancer-targeting molecules. This opens the door for the development of a new class of improved At-211-based TAT drugs.
Contact
Jonathan Burns
University of Alabama at Birmingham
burnsjon@uab.edu
Sherry Yennello
Texas A&M University
yennello@tamu.edu
Funding
This work was supported by the Department of Energy (DOE) Office of Science, Office of Isotope R&D and Production and the DOE Established Program to Stimulate Competitive Research (EPSCoR). Members of the team were also supported by the National Science Foundation, the DOE Office of Science Office of Isotope R&D and Production, Texas A&M University, the Texas A&M System National Laboratories Office through collaboration with Los Alamos National Laboratory, and the Texas A&M Nuclear Solutions Institute. Computational aspects of this work were made possible in part by a grant of high-performance computing resources and technical support from the Alabama Supercomputer Authority.
Publications
Perera, J.S., et al., Probing the Redox Reactivity of Alkyl Bound Astatine: A Study on the Formation and Cleavage of a Stable At-C Bond. Inorganic Chemistry 64, 911 (2025). [DOI: 10.1021/acs.inorgchem.4c04081]