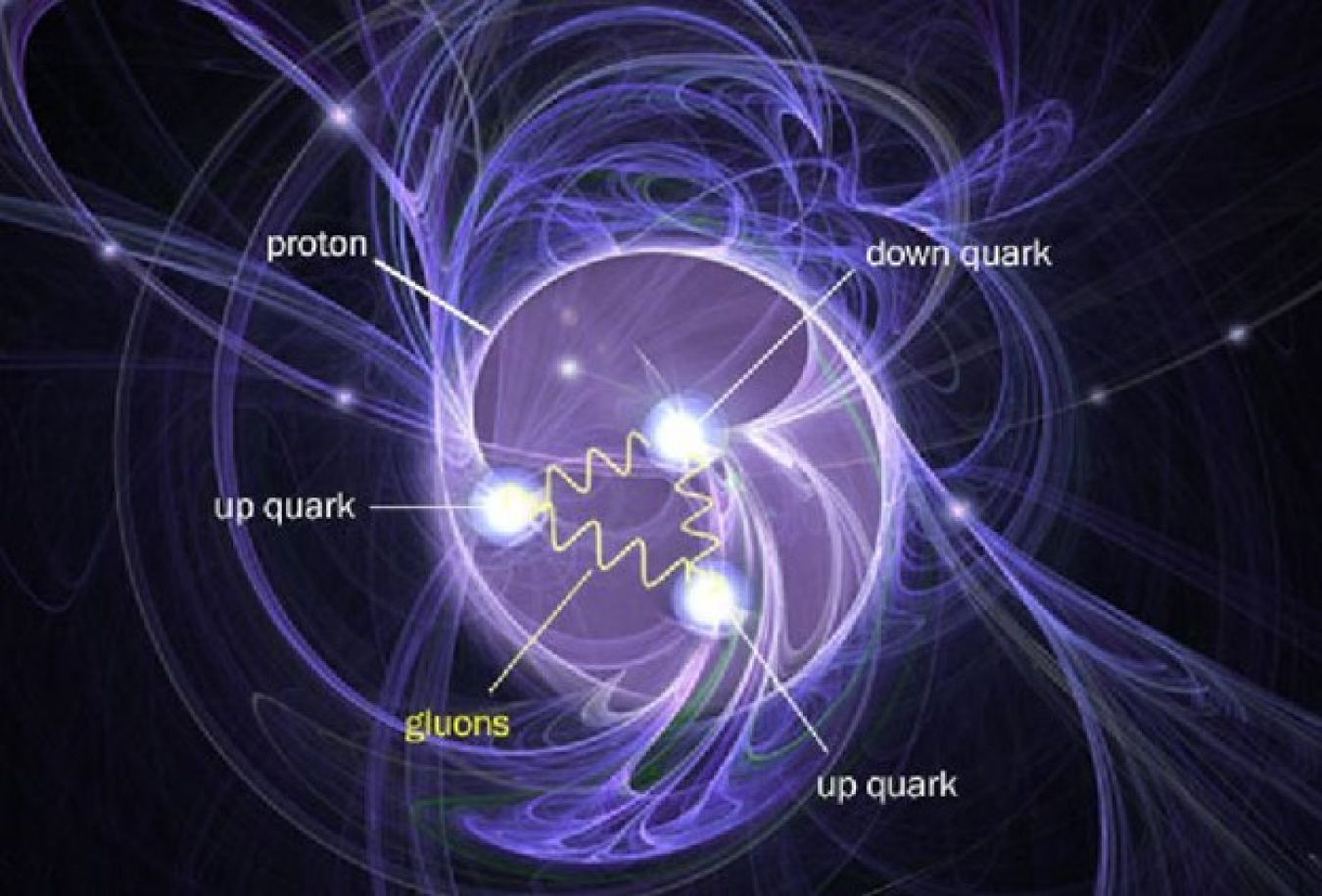
The proton is a subatomic particle with a positive electrical charge. They are found in every atomic nucleus of every element.
In almost every element, protons are accompanied by neutrons. The only exception is the nucleus of the simplest element, hydrogen. Hydrogen contains only a single proton and no neutrons.
The number of protons in an atom determines its atomic number in the Periodic Table of Elements. The number of protons plus the number of neutrons in a nucleus determines an element’s “baryon number,” which is nearly equal to the atomic mass of that element. Protons have a slightly smaller mass than neutrons. That’s why atomic mass numbers aren’t whole numbers. For example, carbon has six protons and six neutrons for an atomic mass of 12.011 “atomic mass units.”
Because they are part of the nucleus, scientists sometimes refer to protons and neutrons as nucleons. Scientists also refer to protons and neutrons as hadrons. Hadron is a term for a composite subatomic particle – a particle that consists of even smaller particles. Specifically, hadrons are made of two or more quarks held together by the strong interaction force, one of the fundamental forces in the universe. Protons contain two up quarks and one down quark, while neutrons contain one up quark and two down quarks. These quarks are called “valence” quarks to contrast them with the “sea” quarks, which constantly pop in and out of existence inside protons and neutrons.
Unlike neutrons, protons are stable. By stable, scientists mean that free protons—protons that are not connected to neutrons in the nucleus—do not break down, or decay, on their own. This is different from neutrons, which are also made up of smaller particles but break down due to radioactive decay. In fact, protons are the only stable type of subatomic particle that is made of even smaller particles.
DOE Office of Science: Contributions to Subatomic Particle Research
The DOE Office of Nuclear Physics in the Office of Science supports research to understand all forms of nuclear matter and the subatomic particles that make up atomic nuclei. This research includes unraveling previously unknown properties of atoms and the subatomic particles they are composed of in their natural state. This information could have important applications in medicine, commerce, and national defense. Another area of study is understanding precisely how nuclei are structured depending on the number of protons and neutrons inside them. Other research focuses on heating nuclei to the temperature of the early universe to understand how they condensed out of the quark-gluon soup that existed just after the Big Bang.
Fast Facts
- Learn more with this Interactive Periodic Table of Elements.
- Scientists are getting closer to measuring the radius of the proton.
- Many things in nature are symmetrical, but not protons. Learn more about proton asymmetry and what it means.
- Learn the technical details of the proton and other subatomic particles at the interactive Particle Data Group site.
Resources
- The Department of Energy Office of Science’s Nuclear Physics program
- The U.S. Particle Physics research consortium
- Science research highlight: How stiff is the proton?
- Science research highlight: The proton antimatter imbalance
Scientific terms can be confusing. DOE Explains offers straightforward explanations of key words and concepts in fundamental science. It also describes how these concepts apply to the work that the Department of Energy’s Office of Science conducts as it helps the United States excel in research across the scientific spectrum.

