A research team from Pacific Northwest National Laboratory investigates how potassium in biomass feedstocks poisons a catalyst.
August 11, 2022
Author: Dr. Asanga Padmaperuma
Senior Research Chemist in the Energy Processes and Materials Division at Pacific Northwest National Laboratory
Read Asanga's bio ►
Meet the other bloggers ►
Return to Bioprose blog ►
A research team from Pacific Northwest National Laboratory investigates how potassium in biomass feedstocks poisons a catalyst. Their discovery allowed researchers to better understand catalyst health and regeneration during the conversion of feedstocks into biofuels and bioproducts.
The creation of biofuels involves a little bit of nature’s magic. A catalyst, like mixed transition metal oxide, enables a chemical reaction that transforms agricultural waste or a similar renewable feedstock into clean biofuel capable of powering vehicles, airplanes, ships and other engines.
While catalysts are the magicians in that process, they naturally deactivate—or stop working—at varying rates over time and replacing them is costly.
Metals like iron and potassium that are inherent in certain biomass feedstocks interact with the catalyst, poisoning it and causing loss of catalyst function. The rapid catalyst deactivation causes unexpected interruption of operations and the need to replace the catalyst more frequently.
A research team, led by Pacific Northwest National Laboratory (PNNL) bioenergy scientist Huamin Wang and supported by Oak Ridge National Laboratory and the National Renewable Energy Laboratory, recently unlocked the mystery of catalyst poisoning by these metals. Their findings have significant implications for biofuels production.

PNNL researcher Huamin Wang evaluates a catalyst poisoned by potassium. Photo: Andrea Starr | Pacific Northwest National Laboratory
Results from the team’s study, which was supported by the U.S. Department of Energy (DOE) Bioenergy Technologies Office, were published in ACS Catalysis.
Studying Deactivation to Improve Catalyst Regeneration
To understand why some metals found in biomass feedstocks accelerate catalyst deactivation, the team needed to find the right catalyst for the experiment. They ultimately selected a bifunctional catalyst made of platinum/titanium dioxide (Pt/TiO2). This catalyst contains different active sites—some for oxygen removal and others catalyze carbon bond arrangement, both of which are critical for producing quality, cost-competitive bio-oils. These catalysts create multiple chemical reactions that are critical for catalytic fast pyrolysis (CFP), a process which involves rapidly heating biomass and upgrading the produced vapor by the catalyst.
Pick Your Poison
The researchers focused their study on potassium, a common alkali metal found in biomass feedstocks since previous analysis of deactivated catalysts after CFP of woody biomass feedstock revealed potassium accumulation on the catalysts’ surface.
The research team rigorously simulated catalyst poisoning at different potassium levels to trigger deactivation during industrial operations. They then used a variety of microscopic and spectroscopic capabilities to analyze the catalysts and conduct kinetic measurements to determine how the catalyst’s ability to catalyze chemical reaction changes with the introduction of potassium.
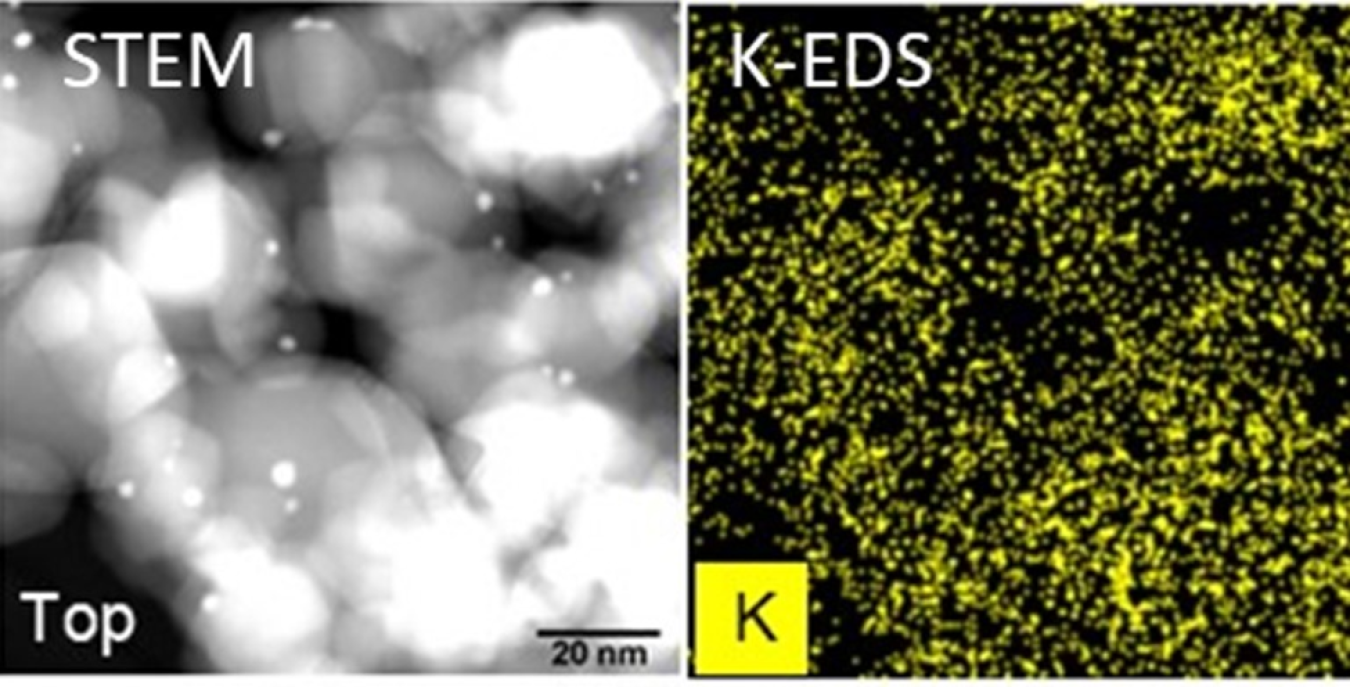
Scanning transmission electron microscope image of an energy-dispersive x-ray diffraction map of potassium distribution on the catalyst. (Photo submitted by Huamin Wang, originally from team member Kinga A. Unocic, Oak Ridge National Laboratory)
Regeneration Remedy
Researchers discovered that the potassium completely deactivated the catalyst with preference at certain sites, and the preference varied with the potassium content. The higher the potassium, the greater the deactivation. The team was also able to document how potassium poisons different types of active sites on the catalyst.
Ultimately, they found potassium poisoning could be substantially mitigated with a developed regeneration method—a water washing process—that can successfully remove most of the loaded potassium, restoring more than 90 percent of the catalytic activities.
Reducing Risk and Improving Catalyst Design
The results of these studies have provided new insights for the bioenergy industry that will foster improved catalyst design and regeneration for longer-lasting catalysts. The studies also created a solid knowledge base for developers of biomass conversion technologies to continue to build upon, making new and innovative conversion technologies less risky to research and develop. The research team’s work also supports accelerated process development that can help industry convert biomass feedstocks commercially, leading to more effective and inexpensive production of biofuels.
Dr. Asanga Padmaperuma

Dr. Asanga Padmaperuma is a Senior Research Chemist in the Energy Processes and Materials Division at Pacific Northwest National Laboratory. He serves as the Laboratory Relationship Manager for the Department of Energy's Bioenergy Technologies Office, which has oversight of research in renewable use of marine, terrestrial, and waste biomass for fuels and chemicals via thermal, biological, and electrocatalytic conversion.
Dr. Padmaperuma also serves as the Technical Leader for the Chemical Conversion team in PNNL's Chemical and Biological Processing Group. Prior to his current assignment, his research focus was on thermochemical, low-temperature catalytic, and electrochemical production of value-added chemicals and fuels from bio-based and waste sources. He also conducted research in design and development of functional molecules for organic electronics and liquid scintillators for radiation detection.
Dr. Padmaperuma completed his B.Sc. in Chemistry at the University of Colombo (Sri Lanka) and obtained his Ph.D. in Organic Polymer Chemistry at the University of Southern California's Loker Hydrocarbon Research Institute. He is a named inventor on several granted patents as well as an author of more than 50 journal articles, book chapters, and public technical reports.
Meet our other bloggers ►
Return to Bioprose blog ►


