Researchers propose a new approach to modeling adsorption processes that affect how pollutants move through soil, water, and rock.
August 4, 2025The Science
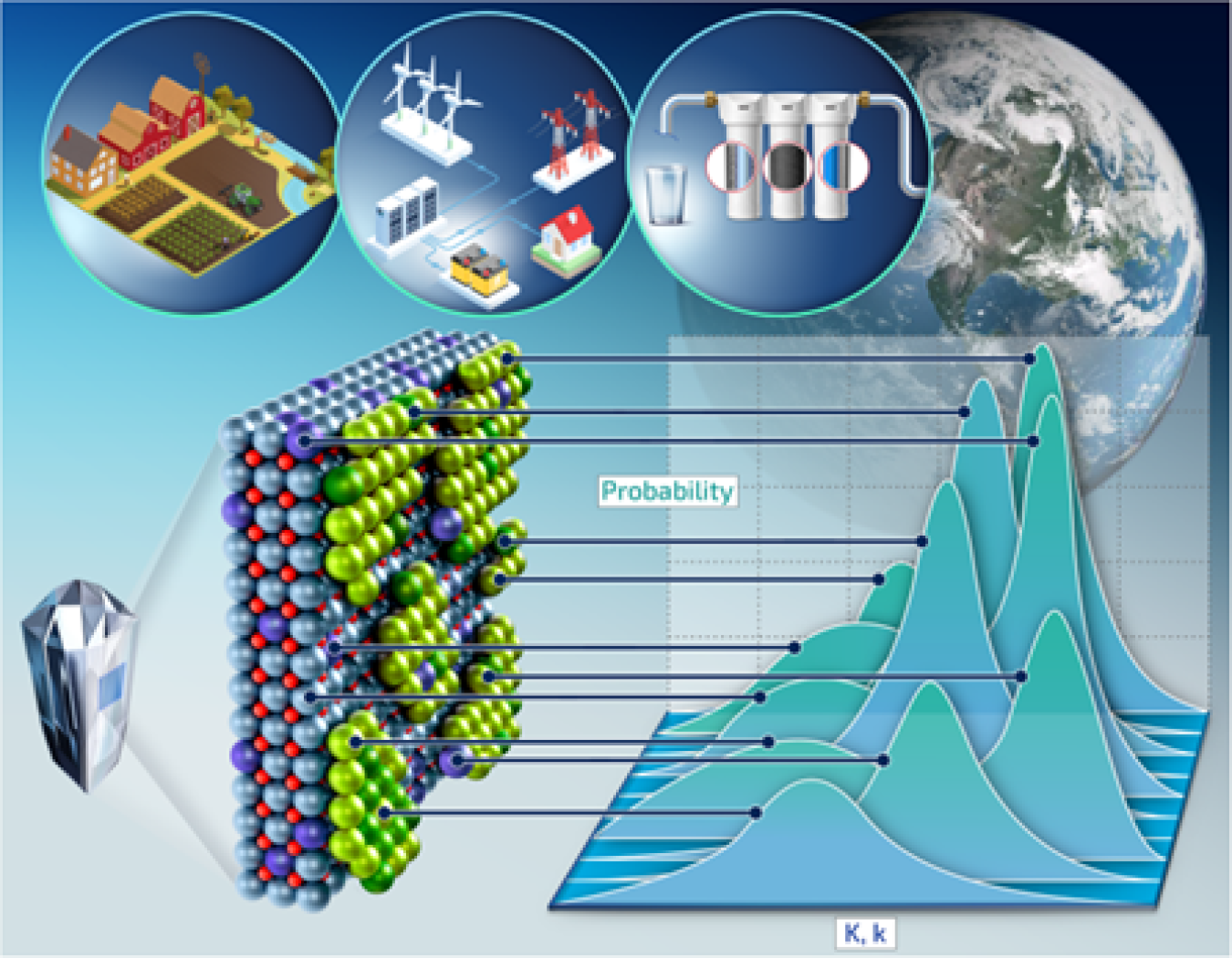
Solid surfaces in contact with water influence how contaminants move through soil and rock and into water and air. These surfaces continuously undergo adsorption and desorption reactions. In these reactions, very thin layers of molecules stick to the surfaces of solid particles. These processes operate at the molecular and nanoscale levels, smaller than bacteria and viruses. Despite the small scale of these adsorption reactions, they control the fate and transport of all chemicals in the environment. This is because solid surfaces are incredibly abundant on Earth, especially in soils, which include a myriad of fine grains. Just a few handfuls of soil contain a reactive surface area equal to a football field. There are roughly trillions of pounds of topsoil on the planet. These surfaces are very complex, so researchers simplify them using models that treat the massive variation in surface chemistries as a small set of specific values derived by simple averaging. But because phenomena in the real world can have a continuum of values, these models oversimplify reality. That’s become increasingly apparent as scientists learn more about these surfaces at the molecular scale. Oversimplifying can mean models fail to predict real-world situations. In this study, researchers proposed a new concept for incorporating molecular-scale knowledge of these solid-water interfaces into larger models. They found that surface properties should be represented with probability distributions instead of specific values. This change would better encompass the variation found in the real world.
The Impact
The method in this research has several benefits for scientists and engineers. It would first capture the chemical reality of heterogeneous surfaces. This is a non-trivial task that scientists have been working on for decades. Second, incorporating this approach will improve environmental fate and transport models. These models describe what happens at sites that store carbon or nuclear waste as well as sites where there have been chemical spills over time scales of hours, days, years, and millennia. They help researchers understand how chemical, biological, or radiological contaminants can seep into the soil and enter groundwater, move into streams, or be transported through the air. This method would also help predict how soil systems evolve. Finally, it would improve the design of electrochemical and catalytic processes, materials used for carbon capture, and other technologies based on interfacial chemistry.
Summary
This work builds on the team’s previous investigations into interfacial chemistry and challenges they encountered when they incorporated molecular-scale observations into continuum-scale models. The previous studies of solid-water interfaces revealed site-dependent reactivities and surface heterogeneity, at all scales, even down to the molecular level. While scientific understanding of the molecular details of surfaces is progressively improving, these details have not yet been incorporated into the widely used continuum-scale models. These models are based on fundamental thermodynamic and kinetic principles developed for chemical systems in a well-mixed state. Therefore, they best represent nice, idealized surfaces that are almost never encountered in reality besides extremely clean laboratory systems.
In this work, the researchers reviewed studies of molecular details of reactive surfaces and the limitations of current continuum-scale models. They illustrate that surfaces cannot be accurately represented by classical kinetics and thermodynamics that best capture clean “idealized” surfaces and well-mixed systems. Instead, they propose a probabilistic approach to formalizing interfacial chemistry to account for the distributions of chemical descriptors, such as various equilibrium constants, in surface reactions.
Contact
Anastasia G. Ilgen
Geochemistry Department, Sandia National Laboratories
agilgen@sandia.gov
Funding
This material is based on work supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Geosciences Program. Support for the researchers also came from the National Science Foundation, the Army Research Office, and the American Chemical Society Petroleum Research Fund.
Publications
Ilgen, A.G., et al., Bridging Molecular-Scale Interfacial Science with Continuum-Scale Models. Nature Communications 15, 5326 (2024). [DOI: 10.1038/s41467-024-49598-y]