Hydrogen is typically produced at relatively low pressures (20–30 bar) and must be compressed prior to transport. Most compressors used today for gaseous hydrogen compression are either positive displacement compressors or centrifugal compressors. Positive displacement compressors can be reciprocating or rotary.
- Reciprocating compressors use a motor with a linear drive to move a piston or a diaphragm back and forth. This motion compresses the hydrogen by reducing the volume it occupies. Reciprocating compressors, sometimes called recips for short, are the most commonly used compressor for applications that require a very high compression ratio.1
- Rotary compressors compress through the rotation of gears, lobes, screws, vanes, or rollers. Hydrogen compression is a challenging application for positive displacement compressors due to the tight tolerances needed to prevent leakage.
- Ionic compressors are similar to reciprocating compressors but use ionic liquids in place of the piston. These compressors do not require bearings and seals, two of the common sources of failure in reciprocating compressors. Ionic compressors are available today at the capacities and pressures required at hydrogen fueling stations.
- Centrifugal compressors are the compressor of choice for pipeline applications due to their high throughput2 and moderate compression ratio. Centrifugal compressors rotate a turbine at very high speeds to compress the gas. Hydrogen centrifugal compressors must operate at tip speeds 3 times faster than that of natural gas compressors to achieve the same compression ratio because of the low molecular weight of hydrogen.
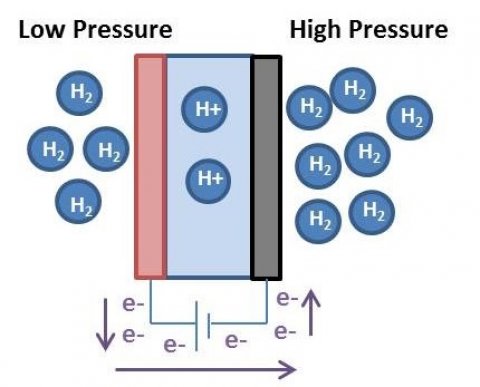
Alternatives to mechanical compression that are currently in the research and development stage include the use of electrochemical reactions, metal hydrides, and ionic liquids. Electrochemical compressors use proton exchange membranes flanked by electrodes and an external power source to drive the dissociation of hydrogen at the anode and its recombination at higher pressures at the cathode.
Metal hydride compressors use metals that form hydrides via exothermic reactions and then release hydrogen at high pressures when heat is applied.
Notes
1 Compression ratio is the ratio of the pressure at the outlet of the compressor over the pressure at the inlet of the compressor. For example a compressor which takes gas at 20 bar at the inlet and compresses it to 200 bar at the outlet has a compression ratio of 10 [200 bar / 20 bar = 10].
2 Compressor throughput is the rate at which a compressor can compress hydrogen given in mass per unit time (e.g., kg/h).