Researchers at the National Energy Technology Laboratory developed a new catalyst for turning CO2 into fuel that is much cheaper and works much better than the platinum used today.
November 14, 2016Watch to learn about a cheap and effective new way to turn CO2 into fuels and chemicals. | Video courtesy of NETL.
A catalyst developed by researchers at the National Energy Technology Laboratory (NETL) is revolutionizing catalyst design -- the way they are studied, how they work, and how much they cost -- and it may help meet the ever-growing demand for energy with minimal environmental impact.
“This is a big deal!” -- Douglas Kauffman, lead researcher
Catalysts make things happen. They speed up chemical reactions and reduce the amount of energy needed to produce useful products. For example, catalysts are used in electrochemical systems to convert carbon dioxide (CO2) emissions into valuable fuels and chemicals. Carbon dioxide conversion is important because it redirects CO2, a major greenhouse gas, away from the atmosphere and into useful products. On an industrial scale, CO2 conversion offers a way to reduce CO2 emissions associated with burning fossil fuels. Catalysts can also convert water into hydrogen, which is an ultra-clean energy source for a variety of applications.
An electrochemical system can be thought of like a battery: it contains negative and positive terminals called electrodes. The negative electrode provides the electrons needed to convert CO2 and produce hydrogen. Just like a battery, an electrochemical system also requires a positive electrode for electricity to flow. The positive electrode completes the circuit by oxidizing water into oxygen. Instead of powering a phone or a light bulb, the circuit does chemistry.
The great thing about CO2 conversion systems is that renewable energy sources, such as wind and solar, can power them. This provides an opportunity to produce “carbon neutral” or “carbon negative” fuels, because no additional CO2 emissions are associated with the power source.
Unfortunately, oxygen formation at the positive electrode traditionally requires very expensive metals, like platinum, which can make electrochemical systems costly to operate. Luckily, NETL researchers have come up with a better way: they developed a nickel-based nanocatalyst that is much cheaper than platinum and works much better.
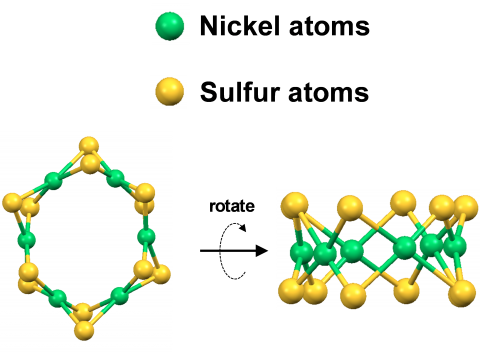
The new NETL catalyst contains six nickel atoms connected to each other with bridging sulfur atoms. Researchers used advanced X-ray techniques to determine that the catalyst’s atoms were arranged in a ring that resembled a crown or tiara. This tiara-like shape provided a uniform atomic structure that allowed researchers to understand its reactivity. They discovered that the catalyst was extremely stable, and it produced oxygen from water even better than platinum.“This is a big deal!” says Douglas Kauffman, lead researcher on the project. “Precious metals are a serious cost-barrier for large-scale deployment of electrochemical systems, and eliminating them from the picture should enable less expensive electrochemical systems.”
NETL researchers hope the new nickel catalyst, and catalysts using other similarly inexpensive materials, will accelerate the installation of large-scale CO2-conversion and hydrogen-generation systems for the production of carbon-friendly, environmentally responsible fuels and chemicals.
Editor’s Note: This post was provided by the National Energy Technology Laboratory, one of the Department of Energy’s 17 National Labs.