Unusual filling of different sub-shells due to quantum confinement leads to a stable superatom that is also highly magnetic.
August 16, 2022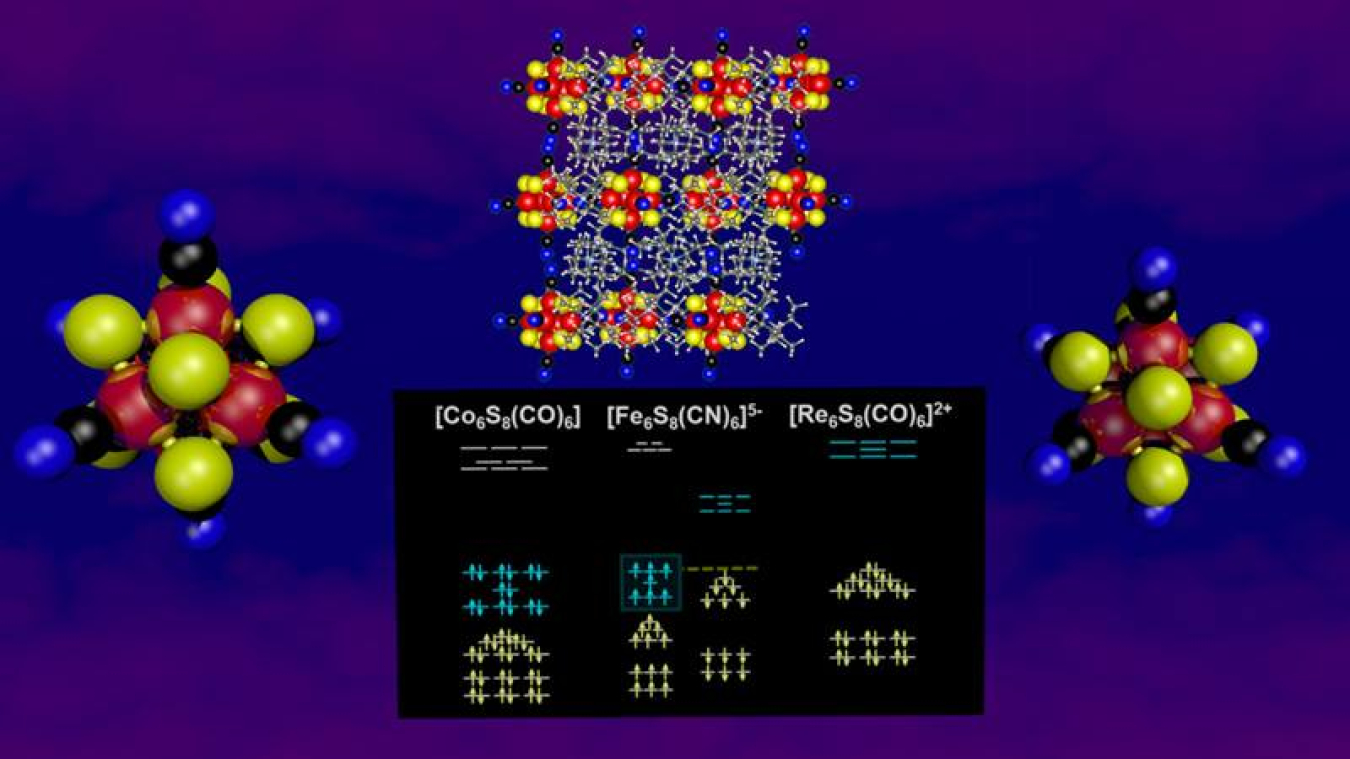
The Science
Industry uses magnetic materials in applications ranging from data storage and cell phones to motors and sensors. Researchers are always looking for new magnetic particles as the technology moves to smaller and smaller scales. In this study, researchers first theoretically predicted, designed, and then synthesized a new, extremely small, thermally stable magnetic nanoparticle. The nanoparticle is designed to be a small symmetric cluster of atoms that acts as a superatom. In superatoms, quantum confinement causes the electronic states of the cluster of atoms to take on an arrangement that mimics the electronic shells and occupancies of a single atom. This gives superatoms unique properties that are rare in nature. In the new nanoparticle, two different electronic subshells are filled; one with 57 electrons and the other with 50 electrons. This dual shell filling produces the energetic stability that enables the superatoms to be synthesized and creates the stable magnetic orderings that give superatoms their unique properties.
The Impact
Researchers can use the new magnetic cluster to make materials with programmable properties. These include electrical conductivity, optical properties, and tunable and switchable magnetic ordering. Researchers can adapt the cluster to accept or donate multiple electrons. This opens the door to multicomponent magnetic solids. The cluster can also be deposited on two dimensional semiconductors. This changes how the semiconductors interact with electrons and allows researchers to tailor semiconductors for new applications.
Summary
Hund’s rule in atomic physics and the Stoner model in solid-state physics describe the traditional mechanisms by which spin magnetic moments arise in atoms and ferromagnetic metals. In both models, valence electrons avoid pairing by occupying half-filled orbitals, which increases the exchange energy and lowers the Coulomb repulsion. In this work, researchers from Virginia Commonwealth University, Columbia University, and Harvard University detailed a new mechanism for the formation of magnetic moments in which the quantum confinement of electrons in clusters splits their electronic structures into distinct subshells with different spin directions and number of available orbitals. The filling of each subshell to reach closed-shell electronic configuration stabilizes the cluster and creates differential spin occupations, i.e., a magnetic moment. This mechanism bears a striking resemblance to the mechanism governing magnetic semiconductors, but it uniquely operates at the nanoscale. Jahn-Teller distortions typically prevent the formation of high spin states in clusters but the dual subshell filling mechanism results in both high electronic stability and a large magnetic moment. This is ideal for applications in spin-based electronics because the electronic structure is inherently spin-polarized, and the magnetic moment arising from filling both subshells is robust against structural distortions and defects.
Contact
Shiv Khanna, Commonwealth Professor
Department of Physics, Virginia Commonwealth University
snkhanna@vcu.edu
Funding
Theoretical work was supported by the Department of Energy Office of Science, Basic Energy Science program. Experimental work was supported by the National Science Foundation Materials Research Science and Engineering Centers program on Precision-Assembled Quantum Materials and by the U.S. Air Force Office of Scientific Research.
Publications
Bista, D., et al., High-Spin Superatom Stabilized by Dual Subshell Filling. Journal of the American Chemical Society 114, 5172 (2022). [DOI: 10.1021/jacs.2c0073102]
Related Links
Spotlights on Recent JACS Publications, Journal of the American Chemical Society
From theory to practice: New stable, magnetic superatom could power innovations in nanomaterials, VCU News

