Like any complex technology, SOFCs are subject to external influences. The deleterious effect of chromium (Cr) vapor has been known since 1996 at least. The high SOFC operating temperature, typically around 800 °C, causes a minute amount of Cr near the surface of stainless steel components to vaporize. The Cr vapor poisons the cathode on the airside of the fuel cell, which reduces the conversion of gaseous oxygen to the oxygen ion (the rate limiting step) and reduces the output of the SOFC.
In a project supported by DOE, Dr. Prabhakar Singh and coworkers have found materials that act as getters for Cr vapors. A getter is a material that removes unwanted components, usually a gas or vapor, from a process. Dr. Singh found that a compound consisting of strontium oxide (SrO) and nickel oxide (NiO) is effective. This compound also is desirable due to its stability, reaction product morphology, and ease of formation and processing.
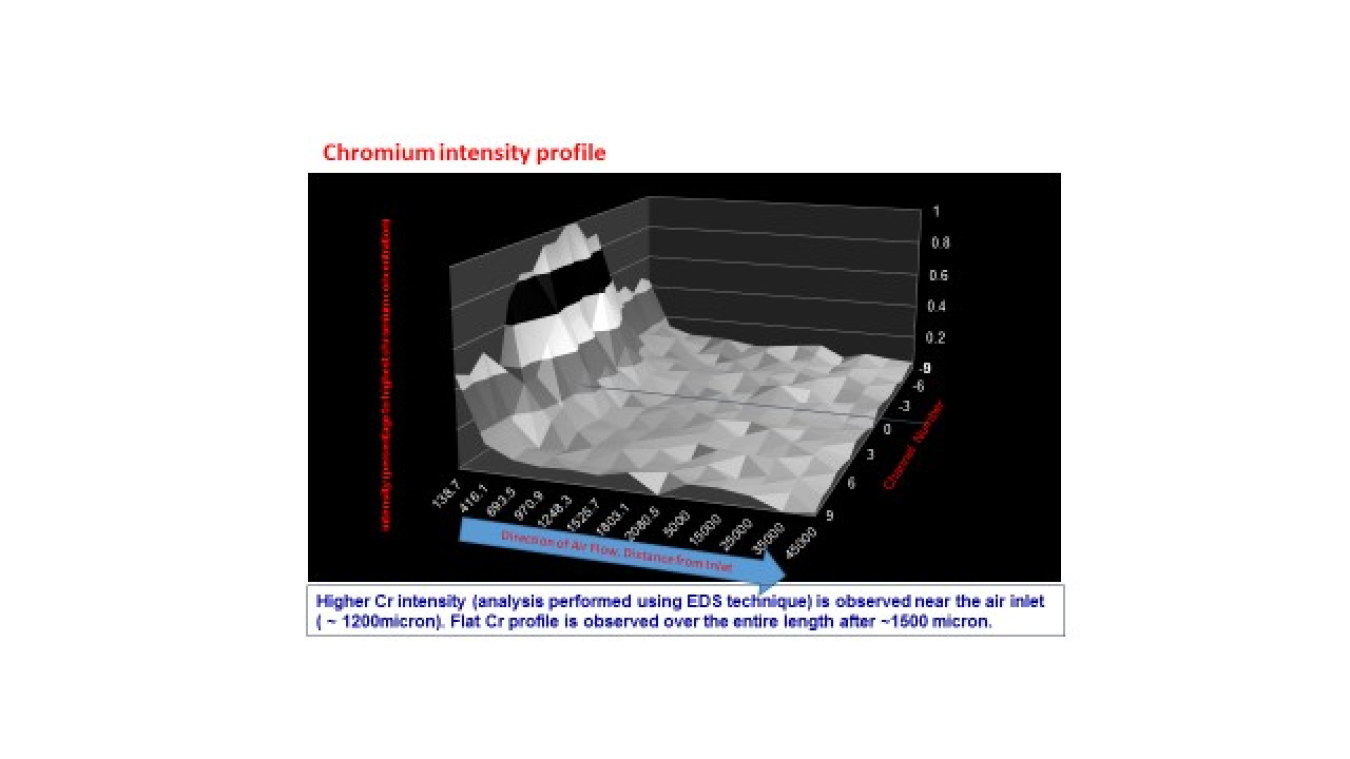
Dr. Singh’s laboratory developed a thermodynamic model of the chemical interactions and used that model to select getter materials. Factors include free energy minimization, stable Cr+3 and Cr+6 containing compound formation as well as Pilling-Bedworth Ratio (the ratio of the unit cell volume of the oxide to that of the base metal), and environmental stability. The model indicated constituents from the alkaline earth and transition metal groups would be useful. SrO containing compound was selected because it also is stable against hydrolysis and capture of water vapor at lower temperatures as well as high temperatures, conditions that occur during thermal cycling and shutdown. Chromium capture occurs in the first 2-3 mm of the getter bed surface.
For more information, click here.