The Hydrogen and Fuel Cell Technologies Office's (HFTO's) metal hydride storage materials research focuses on improving the volumetric and gravimetric capacities, hydrogen adsorption/desorption kinetics, cycle life, and reaction thermodynamics of potential material candidates.
The Hydrogen Storage Engineering Center of Excellence has developed a system projection graph showing a modeled sodium alanate (SAH) system and how it compares against all of DOE's 2020 targets.
Download the final report for the DOE Metal Hydride Center of Excellence.
Technical Overview
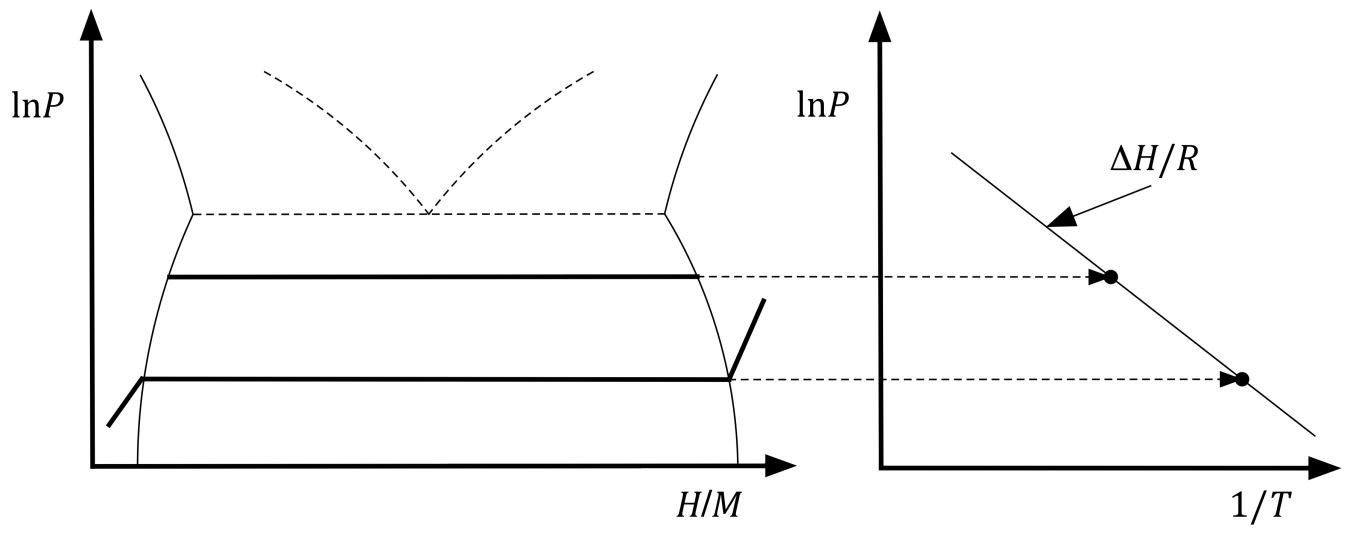
Figure 1. Pressure composition isotherms at left illustrate how the equilibrium pressure at a given temperature can be used to determine the slope of the van't Hoff trace shown on the right.
Metal hydrides (MHx) are the most technologically relevant class of hydrogen storage materials because they can be used in a range of applications including neutron moderation,1 electrochemical cycling,2 thermal storage,3 heat pumps,4 and purification/separation.5 While many alkali or sp metals also form saline or covalent hydrides, the recognition that transition metal hydrides, in particular, are in fact distinct compounds as distinguished from solid solutions of hydrogen is attributable to the band structure calculations of Switendick.6 However, the attribute of relevance where application solutions are sought is the solid solution region of the phase diagram, between metal and hydride phases as shown in Figure 1.
The thermodynamics of hydrogen adsorption/desorption govern their temperature and pressure range of applicability. The enthalpy and entropy over the constant pressure region of the phase diagram can be described by application of the isothermal Gibbs free energy to the van't Hoff equation to yield the linear form:
ln(P) = ΔH/RT - ΔS/R
The thermodynamics over the solid solution region shown schematically can therefore be related to ln(P). The enthalpy ΔH is negative for this category, which reflects the endothermic release of hydrogen for the reaction.
An analysis of the thermodynamic behavior is especially critical for application-specific equilibrium pressure requirements. While a 1 bar equilibrium value is often cited in determining an appropriate value or range of ΔH, polymer electrolyte membrane (PEM) fuel cells may require more than 5 bar pressure. Thus the van't Hoff equation can be used to initially assess the viability of a hydride for a particular application given an operating temperature and pressure range.
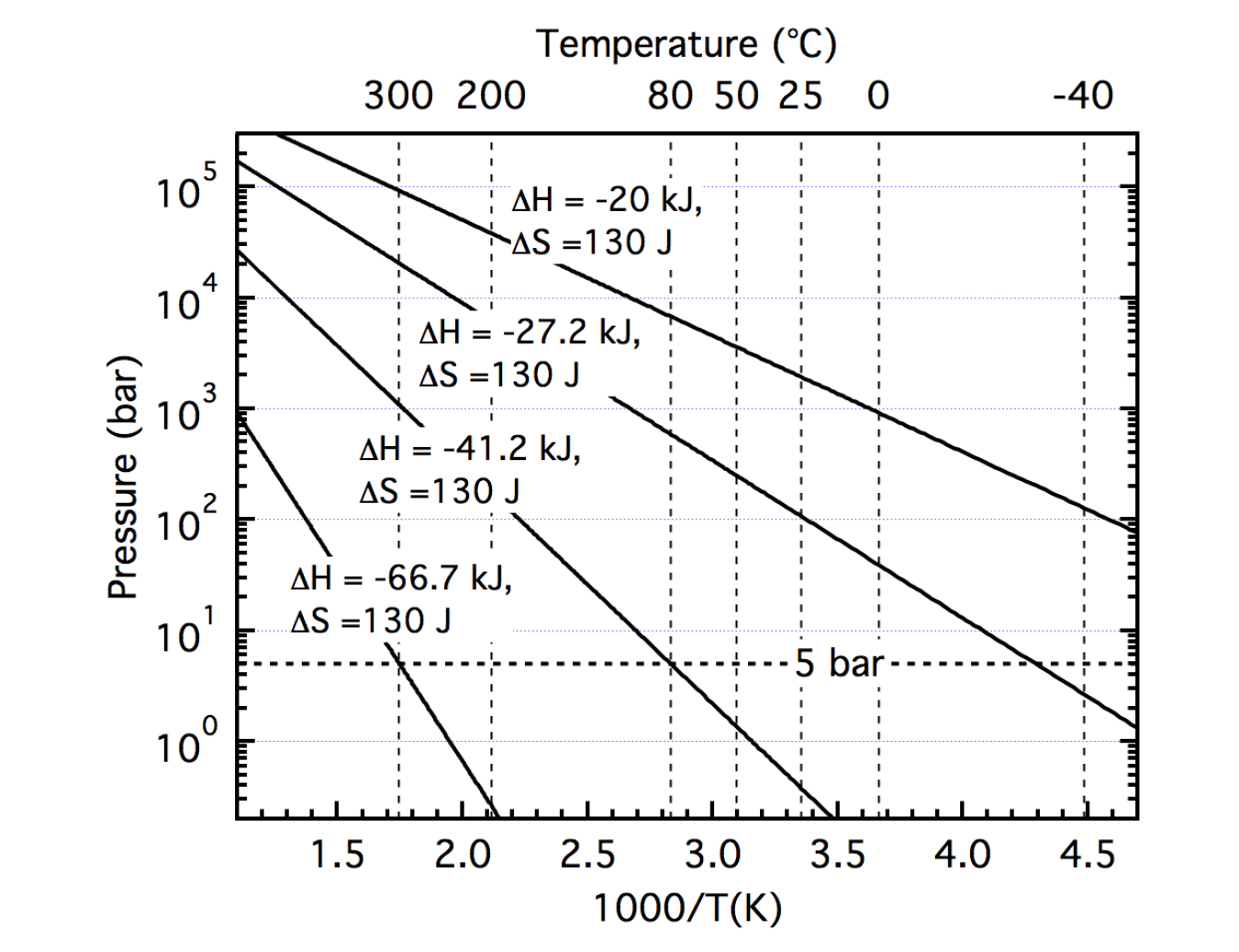
Figure 2. Calculated van't Hoff traces illustrating the range of enthalpies that yield a 5 bar equilibrium pressure assuming a constant entropy.
It is also necessary to know the entropy value as the slope and enthalpy at a particular pressure and temperature can vary depending on the value of ΔS. While it is generally a given that ΔS is dominated by the change in gas phase entropy when hydrogen transforms from diatomic gas to atomic hydrogen into the metal lattice, ΔSH2, Rudman and Sandrock7 noted that a large change in the gas phase entropy value should accompany a change in temperature. However, cognizant of the nearly constant value of ΔS that is determined from empirical work, Oates and Flanagan8 noted that for a given plateau pressure, a nearly constant ΔS would be attributed to a similarly sloped increasing hydride entropy as determined from an Einstein oscillator model. Assuming that the entropy change ΔS is nearly equivalent to the change in gas entropy at ~130 J/mole K, a 5 bar equilibrium pressure over a temperature range of −40°C to 80°C requires an enthalpy between 27 and 41 kJ/mole of hydrogen (Figure 2).
HFTO's metal hydride materials research has more recently focused on so-called complex hydrides that consist typically of alkali or alkaline earth elements that are ionically bonded to a complex anion. The anions themselves can consist of central atoms that are typically transition or main group metals or metalloids (e.g., Fe, Ni, B, Al) or N, to which hydrogen is covalently bonded. As with metal hydrides, the desorption reaction in this series of compounds can be endothermic upon hydrogen release. With the pursuit of higher gravimetric and volumetric hydrogen density materials, the use of complex hydrides that consist of low Z cations offers possibilities for use given a complete dehydrogenation of the stoichiometric hydride.
The invigorated level of effort in investigating this class of materials can be traced back to the original work of Bogdonovic9 when he discovered that Ti-mediated dehydrogenation could be effected in NaAlH4, prior to melting.10 As with a number of complex hydride dehydrogenation reactions, however, the actual reaction pathway and diffusion mechanism seldom consists of a simple route where all of the bound hydrogen is released over a solid solution range that consists solely of hydride and non-hydride phases of these materials.11 In systems where the ionic character of bonding of the complex hydride plays a role, electrostatic forces can dominate and impose limits on the diffusion mechanisms that may be required to effect the atom mobility needed for hydrogen hydrogenation/dehydrogenation within a bulk crystal. Therefore, HFTO focuses on improving not only the volumetric and gravimetric capacities but also the hydrogen adsorption and desorption kinetics and reaction thermodynamics. Long-term cycling effects are also being investigated.
References
- Vetrano, J.B. "Hydrides as neutron moderator and reflector materials." Nuclear Engineering and Design (14:3), 1970; p. 390.
- Young, K-H.; Nei, J. "The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications." Materials (6), 2013; pp. 4574–4608.
- Felderhoff, M.; Bogdanovic, B. "High Temperature Metal Hydrides as Heat Storage Materials for Solar and Related Applications." International Journal of Molecular Sciences (10:1), 2009; pp. 325–344.
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Jr. "Metal hydride hydrogen compressors: A review." International Journal of Hydrogen Energy (39), 2010; pp. 5815–5851.
- Wang, Z.M.; Li, V.; Chan, S.L.I. "Review of alloy membranes/film for hydrogen separation or purification." Journal of Rare Earths (23), 2005; pp. 611–616.
- Switendick, A.C. In Hydrogen in Metals I. Topics in Applied Physics, Vol. 28. G. Alefeld and J. Völkl, eds. Berlin: Springer, 1978.
- Rudman, P.S.; Sandrock, G.D. "Metallurgy of Rechargeable Hydrides." Annual Review of Materials Science (12), 1982; pp. 271–294.
- Oates, W.A.; Flanagan, T.B. "The Solubility of Hydrogen in Transition Metals and their Alloys." Progress in Solid State Chemistry (13), 1981; pp.193–283.
- Bogdanovic, B.; Schwickardi, M.J. "Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials." Journal of Alloys and Compounds (253–254), 1997; pp. 1–9.
- Reilly, J.J.; Wiswall, R.H.; Waide, C.H. Motor Vehicle Storage of Hydrogen Using Metal Hydrides. EPA Report TEC-75/001.
- Callini, E.; Borgschulte, A.; Hugelshofer, C.L.; Ramirez-Cuesta, A.J.; Züttel, A. "The Role of Ti in Alanates and Borohydrides: Catalysis and Metathesis." Journal of Physical Chemistry C (118), 2014; pp. 77−84.

